Forensics: Quantitative tracing of Silicon in CRUD
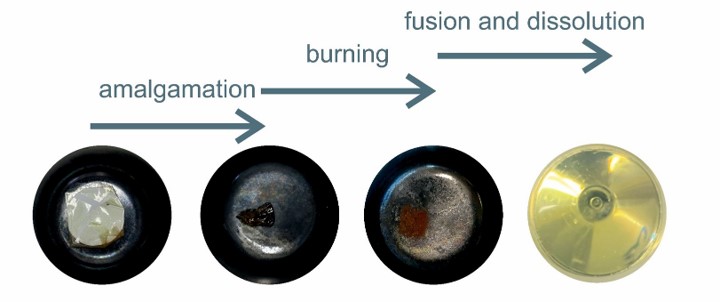
Chalk River Unidentified Deposits (CRUD) are dissolved and suspended solids, product of the corrosion of structural elements in water circuits of reactors [1] in nuclear power plants (NPP). These products can be deposited on the outer layer surface of fuel rods, possibly compromising the thermal efficiency of the rods and potentially leading to fuel failure [2]. Furthermore, migrating CRUD particles potentially cause radioactive contamination hot spots in cooling-water systems [2], as activation products such as 60Co, 54Mn, 65Zn and other radioactive isotopes are often present in crud.
The chemical composition of CRUD is variable since it depends on the composition of the reactor’s structural material, as well as the types of refueling cycles [3]. CRUD has been comprehensively investigated in Swiss nuclear power plants, including detailed quantitative investigations performed in the Hotlab of PSI, on CRUD samples from the nuclear power plant Leibstadt (KKL) [2] using sector field inductively coupled plasma mass spectrometry (SF-ICPMS) measurements of samples dissolved by multi-acid digestion.
Recent investigations have found unexpected but significant Silicon (Si) present in CRUD as Zn2SiO4 and SiO4 [6]. Due to the volatilization of Si, intrinsic of the hydrofluoric acid (HF)-comprising multi-acid digestion, precise quantification of Si content via ICPMS is not possible with the technique utilized in previous studies [2]. The Hotlab is a unique facility in Switzerland for the safe and hazard-free investigation of the properties of highly toxic, radioactive substances. The available sophisticated, extensive laboratory infrastructure, analytical facilities and well-trained personnel allow for the development of analytical strategies such as presented here, which tackle unforeseen obstacles in the characterization of radioactive samples.
Flux-fusion dissolution is an established method for complete dissolution of solid samples [4]. Fusion decomposes the sample by using thermal and chemical energy in order to break up the original mineral phases, converting them into solid forms more readily soluble in aqueous media other than HF [5]. Therefore, the fusion method is ideal to comprehensively study crud compositions including Si.
As a test subject and crud analog, we have devised a mixed reference material named “syntcrud”. Syntcrud is the product of a gravimetrically controlled mixture of inactive, certified reference material NCS DC 28235 and zirconium(IV) oxide (STBJ1668). To address the mineralogical variability of crud, we included in the tests a natural basalt from New Zealand (NZ557) whose major element composition was previously determined [7]. The Syntcrud and basalt (NZ557) were weighted, added to filter and secured with foil; to simulate the conditions of CRUD samples collected using the specialized collection method.
A controlled consumption of filter and foil is achieved by burning the sample assemblage at 600°C in a muffle furnace without the presence of flux (Na2O2:Na2CO3) and under restricted oxygen supply. Without an alkali flux, 600°C is not enough to melt the sample, but sufficient to amalgamate and, finally, burn off foil and filter nearly completely. This avoids dispersion of the sample and prevents contamination, while avoiding the development of insoluble organic components from interaction between foil, filter, sample and flux. The fusion and complete dissolution of the sample is performed subsequent to the amalgamation and burning steps.
The method proposed here was able to reproduce the expected values, in both the Syntcrud and a natural basalt (NZ557), for several elements commonly found in NPP crud. Furthermore, the fusion method should be more efficient in dissolving refractory (i.e. hard-to-dissolve) minerals, possibly comprised in CRUD. In particular, the determined Si content in both materials is identical to the certified or expected values.
This demonstrates that the method presented here is able to reliably provide concentrations of several elements within CRUD, including Si, which was not possible in methods used previously for ICPMS measurement [2].
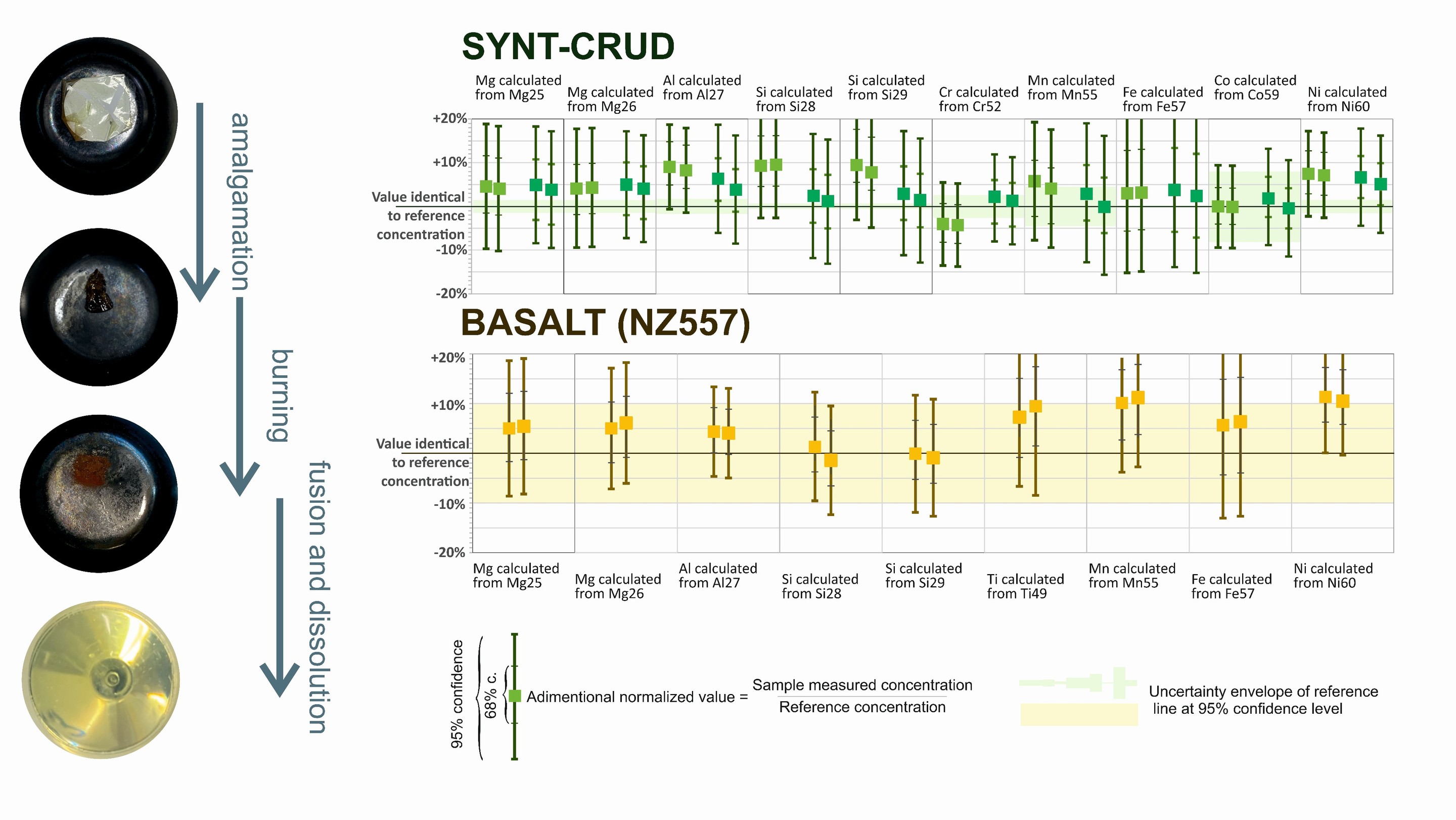
Left side: Four stages of sample. The test assembly of synt-crud powder + filter+ foil in a zirconium crucible vessel before, during and after the pre-fusion burning step and fully dissolved sample in acid after flux-fusion with Na2O2 + NaCO3.
Right side: Measured concentration of Mg, Al, Si, Cr, Mn, Fe, Co, and Ni for two aliquots each of two samples of synt-crud and Mg, Al, Si, Ti, Mn, Fe, and Ni for two aliquots of the basalt (NZ557), normalized to their respective reference values.
The error bars on the individual measurements signifies the uncertainty at 68 and 95% confidence for the measured concentrations, while the 95% confidence uncertainty of the reference value is illustrated by the shaded field in the graphs.
References:
[1] McGrady, J. et al. Development of a microfluidic setup to study the corrosion product deposition in accelerated flow regions. Npj Mater. Degrad. 1, 1–9 (2017).
[2] Günther-Leopold, I., Krois, M., Waldis, J. K., Linder, H. & Abolhassani, S. Investigation of Fuel Crud by Means of ICP-MS and TEM. Procedia Chem. 7, 673–678 (2012).
[3] Kim, W.-S., Nam, S., Chang, S., Kim, H. & Um, W. Removal of Chalk River unidentified deposit (CRUD) radioactive waste by enhanced electrokinetic process. J. Ind. Eng. Chem. 57, 89–96 (2018).
[4] Seelye, F. T. & Rafter, T. A. Low-Temperature Decomposition of Rocks, Ores and Minerals by Sodium Peroxide using Platinum Vessels. Nature 165, 317–317 (1950).
[5] Balaram, V. & Subramanyam, K. S. V. Sample preparation for geochemical analysis: Strategies and significance. Adv. Sample Prep. 1, 100010 (2022).
[6] Robers, L., Le Corre, J.-M. & Prasser, H.-M. Distribution of trace substances in the coolant of a BWR fuel element. Nucl. Eng. Des. 407, 112253 (2023).
[7] Sprung, P., Schuth, S., Münker, C. & Hoke, L. Intraplate volcanism in New Zealand: the role of fossil plume material and variable lithospheric properties. Contrib. Mineral. Petrol. 153, 669–687 (2007).
Rodrigues Barrote, Vitor
PSI
Department Hot Laboratory,
Radioactive Materials Analytics
vitor.barrote@psi.ch
Sprung, Peter
Department Hot Laboratory,
adioactive Materials Analytics
Martin, Mathias
Department Hot Laboratory,
Radioactive Materials Analytics
Diaz, Sasha
Department Hot Laboratory,
Radioactive Materials Analytics